


Next: Homework
Up: Imperfect Gases and Liquids.
Previous: Van der Waals Equation
- 1.
- Virial expansion works for (check all that apply):
- (a)
- gases
- (b)
- liquids
- (c)
- solids
- (d)
- high density
- (e)
- low density
- 2.
- At high temperatures B(T) will be larger for
- (a)
- Larger molecules
- (b)
- Smaller molecules
- 3.
- At high density pressure and free energy in van der Waals
equation behave as:
- (a)
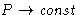 ,
, 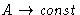
- (b)
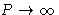 ,
, 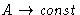
- (c)
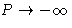 ,
, 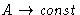
- (d)
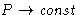 ,
, 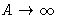
- (e)
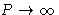 ,
, 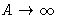
- (f)
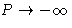 ,
, 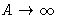
- (g)
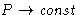 ,
, 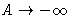
- (h)
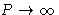 ,
, 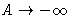
- (i)
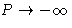 ,
, 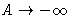
- 4.
- In Boyle point the following is correct (assuming that third and
higher virial terms are negligible):
- (a)
- PV > NkT
- (b)
- PV = NkT
- (c)
- PV < NkT
© 1997
Boris Veytsman
and Michael Kotelyanskii
Thu Sep 18 22:50:29 EDT 1997
![]()
![]()
![]()