Next: Quiz Up: Imperfect Gases and Liquids. Previous: Virial Expansion
![]()
![]()
![]()
Next: Quiz
Up: Imperfect Gases and Liquids.
Previous: Virial Expansion
We know that liquids are (almost) incompressible. So A(N,V,T) should
diverge at ![]() . How can we arrange this?
. How can we arrange this?
Start from virial equation:
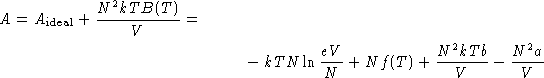
Rearrange the terms:
| |
(8) |
We want an equation, that:
![]()
![]()
| |
(9) |
![]()