


Next: Partition Function and Free
Up: Probabilities of Macroscopic States.
Previous: Canonical Ensemble
Subsections
Let us change the volume reversible . Then 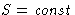 . We define
pressure as
. We define
pressure as
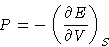
In the definition of temperature we implicitly held 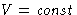 :
:
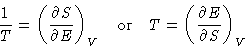
Therefore
| 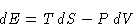 |
(4) |
What do we know about heat from Thermodynamics?
- 1.
- There is ``entropy'' S'. In closed systems
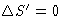 for reversible processes and
for reversible processes and 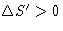 for irreversible
processes (Second Law)
for irreversible
processes (Second Law)
- 2.
- Energy is conserved: for reversible processes in open
systems
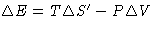 (First Law)
(First Law)
What did we found about our entropy 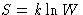 ? Exactly same
things!
? Exactly same
things!
- Conjecture:
- These things are same: S=S'.
- Meaning of the Boltzmann Constant:
- If we defined entropy
as
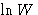 , temperature would have dimension of energy. We measure
temperature differently--and must pay for it by adding a constant!
, temperature would have dimension of energy. We measure
temperature differently--and must pay for it by adding a constant!
We can prove all postulates of thermodynamics from statistics!
© 1997
Boris Veytsman
and Michael Kotelyanskii
Thu Sep 4 21:28:23 EDT 1997
![]()
![]()
![]()
![]() . We define
pressure as
. We define
pressure as
![]()
![]()